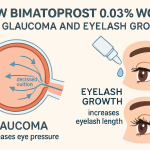
Bimatoprost 0.03% is an eye drop medicine used to reduce intraocular pressure in people with open-angle glaucoma and ocular hypertension, and cosmetically to treat hypotrichosis of the eyelashes. It belongs to a class of medications known as prostaglandin analogues. The blog covers the indications, mechanism of action, administration, contraindications, and side effects of Bimatoprost serum.
Indications
Ophthalmic topical Bimatoprost is a prostaglandin analogue. It was approved by the US FDA (Food and Drug Administration) in 2001 for the treatment of lower intraocular pressure (IOP). Studies show that topical Bimatoprost is among the most effective ophthalmic preparations for lowering IOP in eyes with open-angle glaucoma and ocular hypertension. Eyelash growth is a well-known side effect of Bimatoprost during glaucoma treatment. Later, in 2008, the eye drop received FDA approval to treat hypotrichosis (inadequate eyelashes) due to its effects on longer, darker, and thicker eyelashes. Careprost Bimatoprost ophthalmic solution is often sold over the counter for cosmetic purposes. Bimatoprost is available in the United States at a 0.03% concentration. When used to reduce IOP, the eye drop may be dosed once daily or as your doctor advises.
Once a daily dose of 0.03% bimatoprost ophthalmic solution is applied to the upper eyelashes, it has been studied extensively as an effective formulation for boosting lash growth, with a safety profile and tolerability.
Enhancing eyelash growth with Bimatoprost
The ability of Bimatoprost 0.03% to promote lash growth and reduce IOP was initially observed in patients with glaucoma. A series of clinical trials demonstrated that within 2 weeks of regular application, a greater proportion of follicles were observed in the hair cycle’s anagen (growth) phase and a smaller proportion in the telogen (resting) phase. Therefore, the research study shows that this increased proportion of follicles in the growth phase allows for significantly enhanced eyelash growth with Bimatoprost.
Applying Bimatoprost ophthalmic solution
Bimatoprost ophthalmic solution 0.03% is the most commonly used and is associated with increased eyelash growth with once-daily application. The eyelash growth solution and applicator are commercially available as a bottled solution. Before applying the solution, patients must remove contact lenses. It is not recommended to apply any formulation directly to the eye. For best results, it is advised to apply once every evening before bed. Apply a drop of the product to the applicator brush, then draw a line against the bases of the upper lashes from inwards to outwards. The applicator is to be used with only one eye to prevent contamination. For best results, the solution should be applied for at least sixteen weeks for maximum lash growth.
Usage instructions for the growth of lashes
- Clean your face and remove your makeup and contact lenses before using this ophthalmic solution.
- Be sure to wash your hands before and after applying the ophthalmic product.
- Apply a single drop on the applicator evenly on the upper lash line.
- Be sure to remove any excess solution around the eyes with a tissue or an absorbent cloth.
- Repeat the process for the upper lash line using a sterile applicator.
- Avoid applying the product to the lower lash line.
- Apply 1 drop to each adult’s upper eyelid every night for eyelash growth.
Side effects
Numerous studies have demonstrated the high safety and tolerability of Bimatoprost when used in the eye or eyelid. The most common adverse effects include pruritus of the eye, conjunctival hyperemia, burning sensation, dry eye syndrome, eye pain, visual disturbances, and foreign body sensation.
Bimatoprost application is also associated with changes in iris pigmentation. This is believed to be due to an increased number of melanosomes.
Contraindications
Bimatoprost ophthalmic solution is contraindicated in patients with a history of bimatoprost hypersensitivity or hypersensitivity to any ingredient in the formulation. The solution may not be appropriate for some cases of closed-angle glaucoma and inflammatory glaucoma. It is necessary to remove contact lenses before application of Careprost Bimatoprost Ophthalmic Solution. One can reinsert contact lenses after fifteen minutes. The safety and effectiveness of the product for hypotrichosis have not been studied in kids and infants younger than five years old. The ophthalmic solution is not approved for people under 18 years of age. Also, it is not recommended for pregnant or breastfeeding women.
Since Bimatoprost is a prescription medicine, it should not be used by anyone other than the individual to whom it was prescribed.